
Spectrometers measure this and concentrations can be determined. The higher the concentration the higher percent of light it absorbs. Concentration is correlated to absorbance of light.
THE IMPORTANCE OF SERIAL DILUTION SERIAL
There are many practical uses for serial dilutions. In serial dilution, the density of cells is reduced in each step so that it is easier to calculate the concentration of the cells in the original solution by. The concentration of Solution A is being halved each time you are diluting it. Then from THAT take 25mL and add it to another 25mL of water. Then you take 25 mL of that solution and put it in another 25 mL of water. Serial dilutions is diluting a solution by half each time. There are others, for instance if you're doing a calibration curve, or something like that, but hopefully this explains the issue somewhat. In the end it make the standard preparation that much easier if you have the two middle concentrations. You might replace the dilute one (.01 M in the example) every day, the intermediate one (0.1M) every 2 weeks, and the concentrated one (1M) every 2 months. You might actually use the low level for the test you run, but the shelf life of a low concentration standard is shorter than that of a higher concentration standard. Say you are making standards for calibration of an instrument. Using a serial dilution you get the same final concentration, but now you only used 27 mL of solvent instead of 999 mL.Īside from saving solvent, other reasons this might be good include: - Available glassware, perhaps you have a lot of 10mL, 50mL, and 100mL volumetric flasks in your lab, but not a lot of 1000mL flasks, the serial dilution may be the only way to get the concentration you want. Nevertheless, the importance of locally optimal designs is in that they provide. Finally, 1 mL of the 0.1 solution and put it in 9mL solvent to get the solution you wanted. Next take 1 mL of the 1M solution and put it in 9 mL solvent (0.1M solution). To perform a serial dilution, a small amount of a well-mixed solution is transferred into a new container and additional water or other solvent * is added to dilute the original solution. Instead you could do a serial dilution: Take 1 mL 10M solution, put it in 9mL solvent to make a 1M solution.

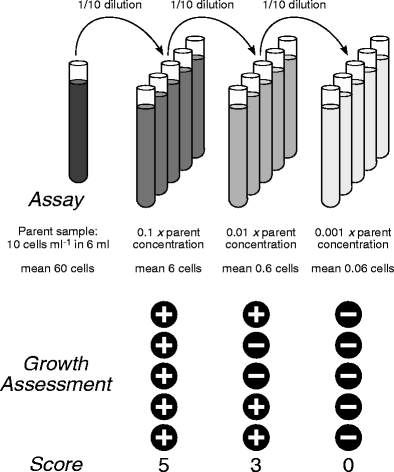
If you did this in one step you would need to take 1 mL of the 10M solution and put it into 999 mL of your solvent. For instance say you have a 10M solution (it doesn't matter what) and you want to make a 10mM solution (.01M). The concentration decreases by the same quantity between each test tube. Usually the dilution factor at each step is constant, resulting in a geometric pro.
THE IMPORTANCE OF SERIAL DILUTION SERIES
Best Answer: It's when you use several steps in a series of dilutions instead of just doing one big dilution. Serial dilutions are created by taking a series of dilutions of a stock solution. erial dilution is the stepwise dilution of a substance in solution.


 0 kommentar(er)
0 kommentar(er)
